Abstract
Background: Follicular lymphoma (FL) is generally incurable with chemoimmunotherapy, and the 15-20% of patients who relapse shortly after therapy have inferior overall survival. Oral small molecule targeted agents are active in FL, but typically fail to induce deep responses as monotherapy and often require extended durations when given in combination. We have previously shown ViPOR to be safe and effective in patients with relapsed/refractory (R/R) aggressive B-cell lymphomas (Melani et al. Blood. 2020). Herein, we present data for all R/R FL pts treated in the dose-escalation and expansion cohorts of the ongoing phase 1b/2 ViPOR study.
Methods: R/R FL pts, excluding grade (G) 3B, with adequate organ function were eligible. Pts were treated with 4 dose-levels (DLs) of venetoclax (VEN) (200mg, 400mg, 600mg, and 800mg) PO D2-14 (starts cycle (C) 2 for DL1) during phase 1 to identify the maximally tolerated dose (MTD) to be used in the phase 2 expansion cohort. Within each DL, a 12d initial ramp-up of VEN was given along with fixed-dose ibrutinib 560mg PO D1-14, prednisone 100mg PO D1-7, obinutuzumab 1000mg IV D1-2, and lenalidomide 15mg PO D1-14. Up to 6C of ViPOR q21d was given without maintenance. TLS, G-CSF, and PCP prophylaxis (ppx) was given to all pts and VTE ppx was per investigator discretion. Baseline CT, PET, BM, and tumor biopsy was performed with CT scans after C1, 2, 4, and 6 and PET after C6 or at time of suspected CR. Surveillance CT was performed q3m for 1y, q4m x 1y, q6m x 1y, then annually x 2y. Minimal residual disease (MRD) was assessed in plasma ctDNA using Adaptive clonoSEQ at baseline, prior to each cycle, and at each follow-up.
Results: 30 pts with R/R FL were enrolled; 8 in phase 1 and 22 in phase 2. Histologic grade was G1-2 in 77% and G3A in 23%. Median age was 63y (range 34-85), with stage III/IV disease in 90%, elevated LDH in 50%, and bulky tumor >10cm in 10%. FLIPI score was low-, intermediate-, and high-risk in 10%, 20%, and 70%, respectively. Median prior therapies were 2 (range 1-6), with 23% of pts refractory (i.e., <PR) to last therapy and 67% of pts high-risk per POD24.
Based on previously presented phase 1 data, VEN 800mg was selected for use in the phase 2 FL expansion cohort. Heme AEs were most common and included G3-4 (% cycles) thrombocytopenia (25%), neutropenia (17%), and anemia (5%). G-CSF was given in 100% of pts and 94% of cycles with 1 case of febrile neutropenia across 163 cycles. The only non-heme G3-4 AE occurring in >10% pts was hypokalemia (13%), with any grade hypokalemia requiring replacement seen in 77% pts. Other common non-heme AEs (% pts) included diarrhea (67%), nausea (53%), and rash (43%). Any grade a.fib occurred in 13% with VTE in 3% and no major bleeding observed. No events of TLS or treatment-related mortality occurred. Dose reductions and delays occurred in 9% of cycles each and 76% pts completed all 6C of planned therapy.
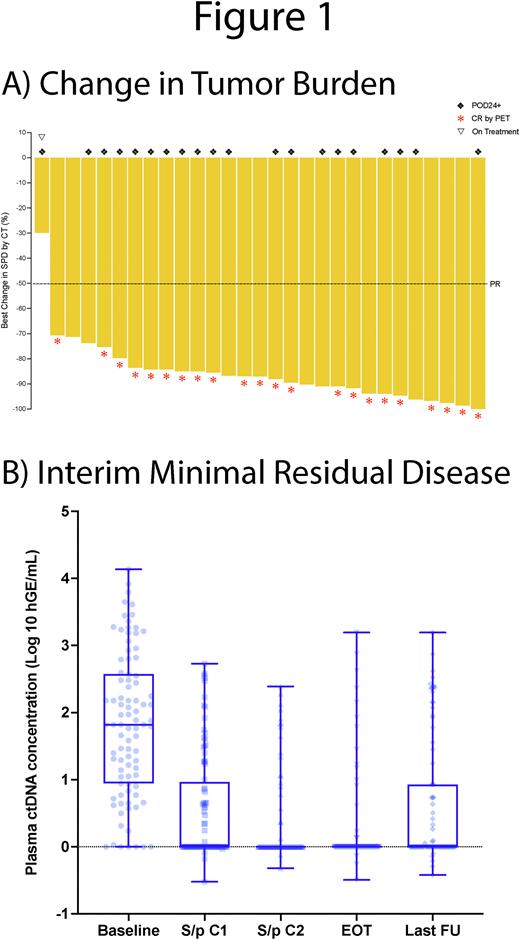
Of 30 total pts, 29 completed at least 1C of therapy with restaging CT and tumor reduction occurred in 100% of pts overall (Fig 1A). Of 28 evaluable pts off therapy, ORR and CR rate were 100% (28/28) and 79% (22/28), respectively, with a CR rate of 79% (15/19) in pts with POD24 after frontline therapy. CR rate was 71% (15/21) and 100% (7/7) in G1-2 and G3A FL, respectively. A calibrating VDJ rearrangement for MRD analysis was identified in 96% (23/24) pts from baseline FF (N=13), FFPE (N=7), or plasma (N=3). Interim MRD in plasma was negative in 27%, 59%, and 73% of pts after C1, after C2, and at end of therapy (EOT), respectively (Fig 1B). In 17 pts in CR by EOT PET with available MRD, 88% were MRD negative at EOT. With a median f/u of 27.3m, median TTR and DOR was 0.66m and 35.6m, respectively, with 50% (11/22) CRs ongoing from 3.2-44.5m. PFS and OS at 2y was 50.9% and 73.3%, respectively, overall; with no significant difference in 2y PFS (39.4% vs 56.6%, P=0.41) or OS (67.5% vs 74.5%, P=0.78) in POD24- vs POD24+ pts. In PET CR pts at EOT, MRD was predictive of PFS with a median PFS of 33.8m vs 5.7m from EOT in MRD- vs MRD+ pts, respectively (P=0.0002).
Conclusion: ViPOR is safe in R/R FL with heme AEs most common and rare febrile neutropenia or severe infections when given with G-CSF prophylaxis. Time-limited, cyclic dosing also resulted in less rash and neutropenia compared to continuous regimens. Fixed duration ViPOR without maintenance induced a high rate of MRD- CRs, including high-risk pts per POD24, with a median DOR of nearly 3y. In PET CR pts at EOT, MRD negativity led to significantly longer PFS.
Disclosures
No relevant conflicts of interest to declare.
OffLabel Disclosure:
ViPOR is not approved for use in FL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal